Rapid Test
Green Spring EN
GREEN SPRING
Rapid Test
Advantages
• BfArM Zulassung
• easy to handle
• fast and reliable test results in only 15 minutes
• 4 sampling methods
• Room temperature storage
• no cross-reactivity with different Coronaviren wie MERS, 229E, NL63, KHU1 oder Influenza A, B
Sensitivity 96,77%
describes how well a test actually detects infected persons and how many infected persons pass through its meshes. In statistics, this is referred to as false-negative results. The closer the value is to 100 percent, the more reliably the test detects all infected persons.
specificity 100,00%
describes how rarely a test falsely classifies people as infected. This is about the statistically false-positive results. Again, the closer the value is to 100 per cent, the better.
Purpose of use
The Green Spring® SARS-CoV-2 Antigen Rapid Test is for the rapid qualitative detection of SARS-CoV-2 nucleocapsid protein antigen in human saliva, nasal, nasopharyngeal or throat swab specimens. The results are used for the detection of SARS-CoV-2 antigen. The antigen is generally detectable in upper respiratory tract specimens during the acute phase of infections. Positive results do not rule out bacterial infection or co-infection with other viruses. The pathogen detected may not be the sole cause of the disease.
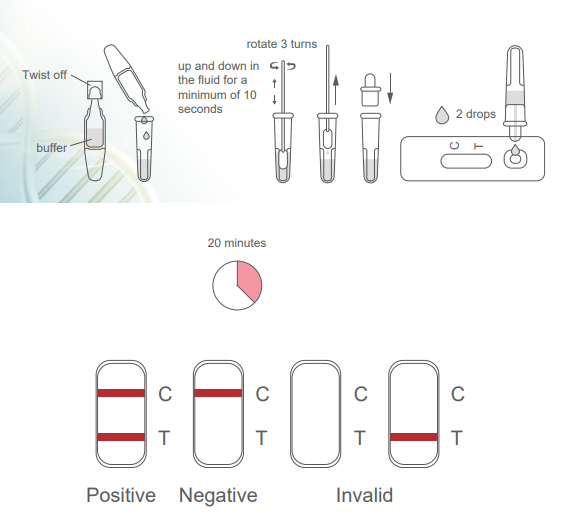
Principle of testing
The Green Spring® SARS-CoV-2 Antigen Rapid Test is a qualitative, membrane-based immunoassay for the detection of SARS-CoV-2 nucleocapsid protein antigens. The test line region is coated with SARS-CoV-2 antibody. The sample reacts with the SARS-CoV-2 antibody in the test line region. If the sample contains SARS-CoV-2 antigens, a coloured line appears in the test line area (T) as a relevant result. As a procedural control, a coloured line appears in the control line area (C), indicating that the correct volume of sample has been added and membrane wetting has occurred correctly.
BfArM listet and proof passed by Paul-Ehrlich-Institut – listet and refundable